How does the rate of severe local skin reactions impact your cosmetically conscious patients' treatment compliance?
Not an actual patient. Results may vary.
Proposed mechanism of action
KLISYRI®: An innovative topical therapy for AK1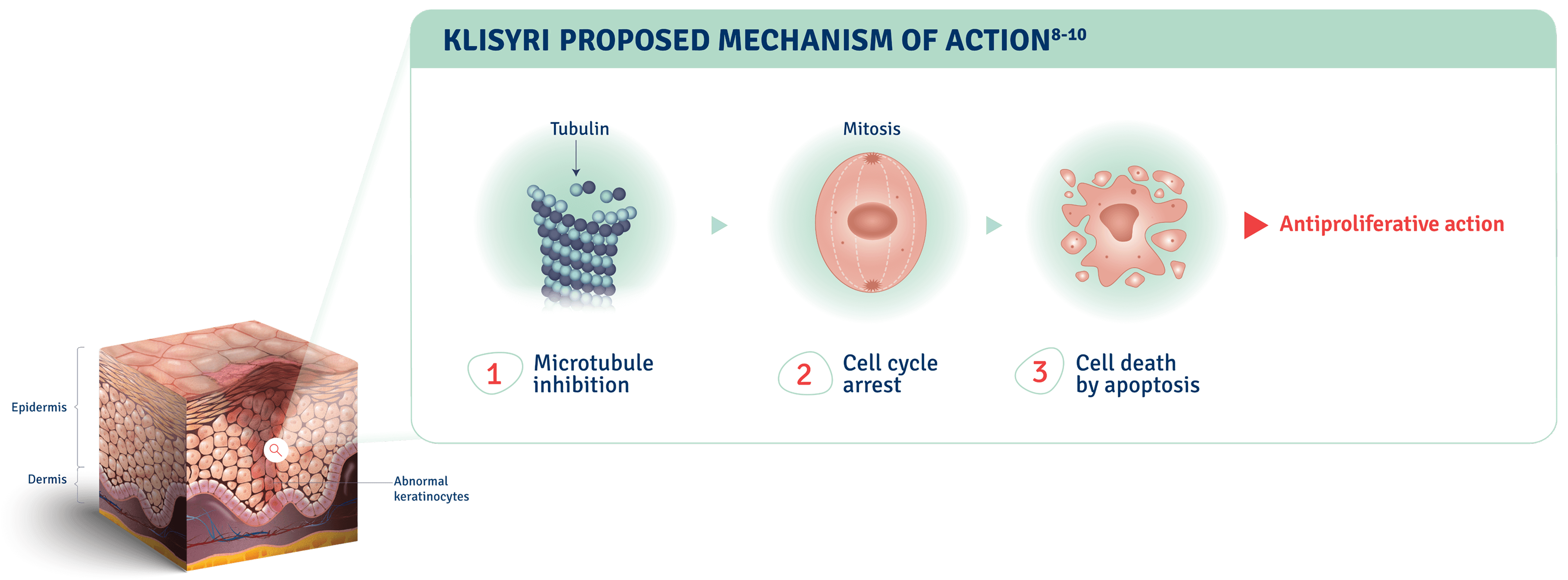
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of face or scalp.1
See Microtubule Inhibition in ActionThe mechanism of action of KLISYRI for the topical treatment of actinic keratosis is unknown.1
AK: actinic keratosis.


KLISYRI is a microtubule inhibitor indicated for the topical field treatment of actinic keratosis on the face or scalp.
IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS Ophthalmic Adverse ReactionsKLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
ADVERSE REACTIONSThe most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
Please see full Prescribing Information.




